 |
DATA ARTICLE | SHORT  |
A chemistry and microbiology data set for meltwater rivers in south-western Greenland (2017–2021)
Sanne M. Moedt1  , Kristian K. Kjeldsen1, Anders R. Johnsen2
, Kristian K. Kjeldsen1, Anders R. Johnsen2  , Andreas P. Ahlstrøm1
, Andreas P. Ahlstrøm1  , Christian N. Albers*2
, Christian N. Albers*2 
1Department for Glaciology and Climate, Geological Survey of Denmark and Greenland (GEUS), Copenhagen, Denmark; 2Department for Geochemistry, Geological Survey of Denmark and Greenland (GEUS), Copenhagen, Denmark
Abstract
Meltwater rivers in Greenland transport large quantities of freshwater from the Greenland ice sheet and local glaciers to the ocean, significantly influencing marine ecosystems and global biogeochemical cycles. With accelerating ice melt due to climate change, understanding the biogeochemistry of these rivers is critical. Here, we present a data set providing comprehensive biogeochemistry data from 28 meltwater rivers in south-western Greenland. Spanning a period from 2017 to 2021, it includes data on nutrients and other ions, trace metals, sediment, radio and water isotopes, microbiology and cyanotoxins, sampled during field campaigns in June and August–September. This data set offers valuable insights for research on glacial meltwater, biogeochemistry and microbiology, addressing key knowledge gaps in these fields.
Citation: Moedt et al. 2025: GEUS Bulletin 59. 8391. https://doi.org/10.34194/gn7zyz56
Copyright: GEUS Bulletin (eISSN: 2597-2154) is an open access, peer-reviewed journal published by the Geological Survey of Denmark and Greenland (GEUS). This article is distributed under a CC-BY 4.0 licence, permitting free redistribution, and reproduction for any purpose, even commercial, provided proper citation of the original work. Author(s) retain copyright.
Received: 03 Feb 2025; Revised: 28 Mar 2025; Accepted: 15 Apr 2025; Published: 29 May 2025
Competing interests and funding: The authors declare no competing interests.
This study was supported by funding from PROMICE, funded by the Geological Survey of Denmark and Greenland (GEUS) and the Danish Ministry of Climate, Energy and Utilities under the Danish Cooperation for Environment in the Arctic (DANCEA) and the Greenland Integrated Observing System (GIOS) under the Danish Agency for Higher Education and Science. Fieldwork and collection of samples was supported through collaboration with the Government of Greenland (Naalakkersuisut). KKK acknowledges support from the Independent Research Fund Denmark (grant ID 10.46540/3103-00234B).
*Correspondence: cal@geus.dk
Keywords: freshwater chemistry, meltwater, microbiology, trace metals, water chemistry
Abbreviations:
CFU: colony forming units
PES: polyethersulfone
Edited by: Catherine N Jex (GEUS, DK)
Reviewed by: Two independent anonymous reviewers
Tabular abstract
| Geographical coverage | South-western Greenland (60.9–66.5 °N) |
| Temporal coverage | 2017–2021 (field campaigns in June and August–September) |
| Subject(s) | Cryosphere, Hydrology, Soils and Biogeochemistry |
| Data format(s) | Raw and analysed biogeochemical and geographical data as CSV files. All data are available at https://doi.org/10.22008/FK2/D1Y8UY |
| Sample collection and analysis | Water samples taken from well-mixed river sites (n = 28), which were analysed for physical, chemical and microbiological parameters. |
| Parameters | Sediment concentration, ions, trace metals, radio isotopes, water isotopes, counts of total heterotrophic bacteria, coliform bacteria and Enterobacteriaceae, cyanotoxins and geographical parameters. |
| Related publications | N/A |
| Potential application(s) for these data | Assessment of freshwater biogeochemistry from a range of meltwater rivers in south-western Greenland. |
Data collection
The original purpose of the data collection was to explore water composition and quality, which influenced the selection of data and methods to focus on parameters relevant to understanding the chemical and physical properties of the water. This included analysis of sediment content, nutrient levels and trace metals, as well as the use of techniques suitable for assessing both the general composition and specific contaminants in the water.
Brief description of the geographical, geological and climate setting
The 28 study sites, all located in south-western Greenland, were accessed by boat to navigate the various fjord systems. The initial selection of the sites was based on remote sensing and GIS data sets to mainly assess accessibility, abundance and water quality, respectively, as outlined in the works of Ahlstrøm et al. (2018) and Kjeldsen et al. (2019). This included catchment delineation (see Supplementary Fig. SI-1 to SI-3) and river slope generated using a 30 m digital elevation model, while total ice cover within each catchment was determined from a vectorised PROMICE ice mask (Citterio & Ahlstrøm 2013), updated with Sentinel-2 imagery from summer 2018, and the Randolph Glacier Inventory version 6.0 (RGI6.0; Pfeffer et al. 2014), which maps local glaciers and ice caps around the year 2000. For catchments covered by both data sets, the RGI6.0 estimate was used for local glaciers and ice caps, while the updated PROMICE ice mask provided the ice-sheet extent.
Sample collection
Water samples were collected from a total of 28 meltwater rivers (Fig. 1) during field campaigns in June and late August – early September (herein referred to only as September), carried out over a period spanning 2017–2021 (see Supplementary File S1). One location, D34, was sampled twice because of doubts about the correctness of initial radioactivity analyses (2018) for this site. Chemical analyses were therefore included for this site in 2021 while microbiological analyses were not. Water samples were collected from well-mixed areas of the rivers, free from visible animal droppings, typically a few metres from the riverbank. The sampler wore single-use nitrile gloves disinfected with ethanol prior to collection. Some analyses were conducted on the boat using a temporary laboratory setup, allowing for on-site processing of samples.

Fig. 1 Map of south-western Greenland with sample locations. Blue sites include cyanotoxin data, while orange sites do not. Black squares: towns/settlements.
Samples for the analysis of anions, cations, metals, stable water isotopes and cyanobacterial toxins (microcystins and anatoxin-A fumarate) were initially collected in an acid-washed glass bottle and processed within 2 h after sampling. For anions, cations and alkalinity, subsamples were filtered using a 0.45 μm polyethersulfone (PES) filter (Q-max, Frisenette) and transferred to plastic vials that were stored cold until analysis within a few weeks. For trace metals (excluding Hg), nitric acid was added to a 30 mL plastic bottle before sub-sampling to preserve samples. Trace element samples were analysed in both unfiltered and filtered (Q-max PES 0.45 μm) forms to capture potential sediment interactions. Total mercury was measured only in 2021, for which unfiltered and filtered samples were collected in acid-washed, airtight glass vials at the site, with acid added in the laboratory within 1–2 weeks. For stable water isotopes, 1.5 mL subsamples were filtered into glass analysis vials with septa. For microcystins, including nodularin, sodium thiosulfate was added by default to the 100-mL glass bottle prior to use. For anatoxin-A-fumarate (2017 only), the samples were collected directly in the river in a 1 L glass bottle. Water for microbial counts was collected in sterile 50 mL centrifuge tubes, opened 10–20 cm below the water surface to avoid the surface film.
Sample analysis
Trace metals, radio isotopes and cyanotoxins were analysed by a commercial laboratory (Eurofins Miljø A/S; see Table 1 for a complete list). Here, trace metals were analysed according to DS/EN ISO 17294m:2016 ICP-MS. Cyanotoxins were analysed according to ISO 20179 LC-MS/MS for microcystin-LW, microcystin-LR, microcystin-RR, microcystin-YR and nodularin, and LC-MS for Anatoxin A fumarate. Radio isotopes were analysed according to NF EN ISO 10704 Alpha/beta proportion for total alpha and beta activity, and SO 13168:2015 Liquid scintillation for tritium activity. Total indicative doses were then calculated based on these measurements. It is important to note that the detection limits for radioactivity parameters reported by the commercial laboratory can vary from day to day due to variation in background radiation.
Anions and cations were analysed by ion chromatography (Metrohm 819 IC) using a Metrosep A 150/4.0 column for anions. Stable water isotopes (δ18O and δ2H) were analysed on a Picarro isotopic water analyser.
The sediment concentration was determined as an average from three or four 1 L samples, which were each filtered through a 0.45 μm pre-weighed filter. The filter was then dried and weighed again to determine the sediment concentration in mg/L. Next, the sediment was scraped off the filters and analysed for grain-size distribution on a Malvern particle size analyser, using a subsample of at least 30 mg dried sediment. When less than 30 mg dried material was available, the sediment from all replicates was pooled before analysis. When the sediment concentration was less than 10 mg/L, sediment size distribution could not be determined.
Most microbial analyses were carried out on board the boat using sterile pipettes, alcohol-wiped gloves and an alcohol-wiped work surface. Plate counts of heterotrophic, colony-forming units were carried out using Petrifilms (3M Aqua Heterotrophic Count Plate) for undiluted and 10-fold diluted samples, where the dilutions were prepared in filter-sterilised (0.2 μm) river water from the same location. The Petrifilms were incubated in pentaplicates at 22°C (68 h) and 36°C (44 h) in accordance with DS/EN ISO 6222 to the extent possible when using custom-made, mobile incubators powered by 12 V lead-acid batteries. The temperature in each incubator was continuously monitored using a data logger. The incubators were placed on the deck. The 22°C incubator maintained a consistent temperature within the ISO standard range. The 36°C incubator generally complied but occasionally dropped below the range during heavy wind. Plate counts of faecal indicators were carried out using Petrifilm for coliform bacteria (3M Coliform Count Plate) and for Enterobacteriaceae (3M Enterobacteriaceae Count Plate). The Petrifilms were inoculated with undiluted sample and incubated in pentaplicate at 36°C for 24 h according to DS/EN ISO 9308-1. After incubation, the Petrifilms were stored at approximately 5°C and counted within 12 h.
For the 2019–2021 field campaigns, colonies from the coliform petrifilms and selected Enterobacteriaceae petrifilms were restreaked on MacConkey agar in the laboratory and incubated overnight at 37°C to confirm growth at this temperature, and at 44.5°C to test for the presence of thermotolerant (faecal) E. coli. The isolates were identified by partial forward and reverse sequencing of the 16S rRNA gene (27F and 1492R universal primers (Weisburg et al. 1991), Macrogen Europe, 600–900 bp consensus sequences). The closest type material sequence matches were identified by BLASTn searches (Zhang et al. 2000).
Data description and main features
Our data set contains water biogeochemistry data from 28 meltwater rivers in south-western Greenland. The different sample types are accessible as separate CSV files (Supplementary Files S2–S8). The data set is accompanied by a CSV file containing the different sampling sites and their features (Supplementary File S1), a READ_ME text file describing the different data parameters and a PDF file containing three maps with the sites and their catchment delineations and ice cover (S9). Here, we present an overview of the content of the data set.
Table 1 provides an overview of the different sample types collected at each site, the parameters analysed for each sample type, the units in which the parameters are presented and the detection limit for the analytical methods used.
Sediments
The data set includes sediment concentration data (mg/L, Supplementary File S3) from the glacial meltwater rivers, which carry suspended solids produced by the powerful erosion of basal rock as glaciers move across the terrain (Herman et al. 2021). The Greenland ice sheet contributes approximately 8% of the global fluvial export of suspended sediments to the ocean (Overeem et al. 2017). These sediments also influence the colour and characteristics of Greenland’s rivers and lakes, ranging from clear and dark to milky grey or brown (Burpee et al. 2018). Figure 2 shows the total sediment concentration divided into four size fractions: clay (<2 μm), fine silt (2–16 μm), coarse silt (16–63 μm) and sand (>63 μm). Meltwater at most sites had a total sediment concentration of less than 200 mg/L. However, two sites in June, D10 and D11, had a sediment concentration of 444 and 1372 mg/L, respectively. The large differences in sediment concentrations in June and September observed at some sites, underscore the fact that samples need to be taken across the season to estimate an average sediment transport of a specific meltwater river.
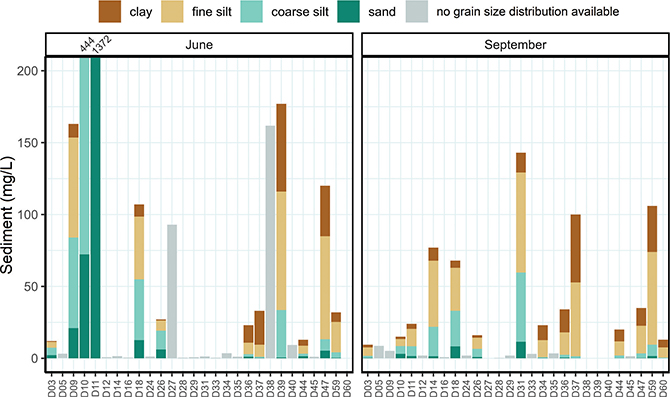
Fig. 2 Sediment concentration (mg/L) and composition (clay, fine silt, coarse silt and sand, available only when sediment content was >10 mg/L) in meltwater rivers in June and September. D38, D39 and D40 were not sampled in September. Note that some low concentration samples may be difficult to distinguish from the background line. To improve readability of the figure, the upper limit of the y-axis was set to 200 mg/L. In June, sites D10 and D11 had a total sediment concentration of 444 and 1372 mg/L, respectively. Grain-size composition was not measured in 2017, hence there are no composition data for sites D27 and D38 in June despite their high sediment concentrations.
Ions and trace metals
Inorganic water constituents, including nutrients, salts and trace metals, were analysed to assess river water characteristics, with particular attention to heavy metals. Southern Greenland’s geology features areas with high metal concentrations (Hawkings et al. 2021), and the relatively unknown geology beneath the Greenland ice sheet (MacGregor et al. 2024) adds uncertainty to the presence of these metals in meltwater rivers. Our data set contains a wide range of inorganic parameters to provide a broad overview of geochemistry in the region.
Supplementary File S2 shows elevated aluminium, nickel and chromium levels in unfiltered samples, likely due to partial extraction of these elements from sediment particles caused by the nitric acid that was added for preservation. In accordance with this, filtering the samples before the addition of nitric acid significantly reduced these concentrations. Figure 3 highlights correlations between sediment concentrations and aluminium and nickel in filtered and unfiltered samples, with aluminium showing consistent fractions extractable with dilute nitric acid, while for nickel this varies across locations. It should be emphasised, that the extraction was not exhaustive and therefore the extractable concentrations cannot be used to conclude on the total amount of trace metals in the sediment particles.
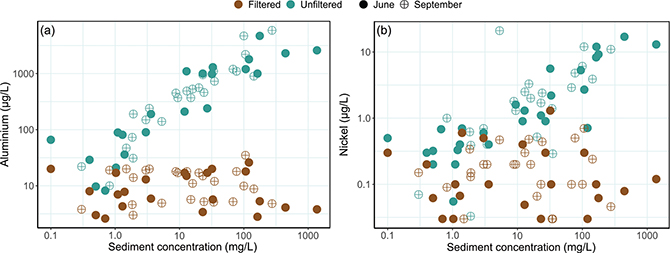
Fig. 3 Concentration of (a) aluminium, and (b) nickel for June and September in unfiltered and filtered samples versus sediment concentration of unfiltered samples. Notice the logarithmic scales. Three values of nickel were below the detection limit (<0.03 μg/L) and for these we report the detection limit only.
Radio isotopes
Some areas in Greenland contain radioactive minerals (Keulen et al. 2014). Therefore, the following radioactivity parameters were included: total indicative dose, tritium and total alpha- and beta-activity (Supplementary File S4). No radioactivity was detected in any of the samples, besides low concentrations in D11 and D34. When D34 was resampled in 2021, values were below the detection limit. As detection limits of radioactivity parameters provided by the commercial laboratory vary from day to day, it is challenging to interpret results near these thresholds.
Water isotopes
Water isotope data (δ¹⁸O and δ²H) are provided in Supplementary File S5. Isotope fractionation occurs during precipitation, where colder temperatures result in fewer heavy isotopes (IAEA 2001). Inland and higher-altitude precipitation also have lighter isotopes, as heavier atoms are preferentially removed during transport. This results in distinct isotope signatures where the Greenland ice sheet has the lightest signature (most negative δ¹⁸O-values), and coastal glaciers, coastal snow and summer rain tend to heavier signatures, with summer rain having the least negative δ¹⁸O-values. These signatures can indicate changes in the relative contributions of the various water sources to the meltwater river at different times.
Microbiology
Heterotrophic bacteria, coliform bacteria and Enterobacteriaceae were detected at varying levels across sites and sampling periods (Fig. 4 and Supplementary File S6). Coliform bacteria and Enterobacteriaceae are generally used as indicators of faecal contamination but belong to the broad Enterobacterales group that includes both faecal indicators and non-faecal environmental species. On 3M coliform Petrifilm, coliforms are identified by acid production, indicated by a faint pink halo and gas production. However, distinguishing gas bubbles and halos under field conditions proved challenging, and all colonies were consequently counted as coliforms. This assumption was validated by replating coliform colonies and selected Enterobacteriaceae colonies from the 2019–2021 field campaigns on MacConkey agar at 37°C, and by 16S rRNA gene sequencing of the colonies (Supplementary File S7). All but two colonies were growth-negative at 44.5°C showing that they were not faecal E. coli. The colonies were identified to the genus level and belonged with few exceptions to common environmental Enterobacterales genera, predominantly Yersinia (47%), Rahnella (19%) and Serratia (16%). One of the thermotolerant colonies was a true Escherichia as expected, whereas the other was a Hafnia, an Enterobacterales genus that generally should not grow under E. coli selective conditions, but is known to sometimes grow at a maximum temperature of 44°C (Greipsson & Priest 1983), which may explain its thermotolerance.

Fig. 4 Bacterial counts as colony forming units (CFU) per mL at the meltwater river sites in June and September for (a) heterotrophs at 22°C, (b) heterotrophs at 36°C, (c) coliform bacteria and (d) Enterobacteriaceae. The detection limit was 0.2 CFU/mL. Notice the differences in y-axis scale.
Cyanotoxins
Supplementary File S8 contains cyanotoxin data from meltwater rivers visited during field campaigns in 2017 and 2018. Cyanobacteria, the dominant photosynthesising bacteria in Arctic freshwater ecosystems, can produce toxins such as microcystins under certain conditions. These data were collected to assess the potential presence of cyanotoxins, as many meltwater rivers are connected to proglacial lakes where cyanobacteria are common. While no cyanotoxins were detected, the detection limit (Table 1) was higher than the concentrations (0.005–0.4 μg/L) reported in a previous study using a highly sensitive immunochemical method that does not differentiate between microcystin and nodularin (Trout-Haney et al. 2016).
Acknowledgements
The authors thank Kisser Thorsøe, Karina Hansen, Bent Hasholt, Danielle Hallé, Øyvind A. Winton and Tina Bundgaard Bech for their technical and analytical support and field assistance, and Erik Palo Jacobsen and the crew on M/S Sterna (Arctic Boat Charter) for safe navigation and valuable assistance in the field. The authors also thank the two anonymous reviewers whose comments improved the manuscript.
Additional information
Author contributions
SMM: Writing – original draft, data curation, visualisation. KKK: Conceptualisation, methodology, investigation, supervision, writing – review & editing. ARJ: Methodology, investigation, writing – review & editing. APA: Conceptualisation, methodology, investigation, writing – review & editing. CNA: Conceptualisation, methodology, investigation, writing – review & editing.
Additional files
Ten additional files, including all data (S1_Sampling_sites.csv, S2_Ions_and_trace_metals.csv, S3_Sediment.csv, S4_Radio_isotopes.csv, S5_Water_isotopes.csv, S6_Microbial_counts.csv, S7_Coliform_Enterobact_16rRNA_ID.csv, S8_Cyanotoxins.csv) a Readme file (READ_ME.txt) and Supplementary Figures S1, 2, 3 (Supplementary_Figure_SI_1_3) are available at: https://doi.org/10.22008/FK2/D1Y8UY.