RESEARCH ARTICLE
Characterising brines in deep Mesozoic sandstone reservoirs, Denmark
Hanne D. Holmslykke*1, Niels H. Schovsbo1, Lars Kristensen1, Rikke Weibel1 and Lars Henrik Nielsen1
*Corresponding author: Hanne D. Holmslykke | E-mail: [email protected]
1Geological Survey of Denmark and Greenland (GEUS), Øster Voldgade 10, DK-1350, Copenhagen K, Denmark
GEUS Bulletin Vol 43 | e2019430104 | Published online: 17 July 2019
https://doi.org/10.34194/GEUSB-201943-01-04
The Danish subsurface contains several sandstone units, which represent a large geothermal resource (Vosgerau et al. 2016). Currently, only three geothermal plants are operating in Denmark, but several exploration licences are expected to be awarded in 2019. Geothermal energy is exploited from deeply buried porous sandstones by bringing warm formation water (brine) to the surface, extracting the heat and returning the cooled water to the same sandstones. The reduced temperature of the brine during this process implies a risk of scaling, which may reduce reservoir permeability and hence injectivity. Predicting the chemical composition of formation waters, however, could help to reduce the risk associated with scaling in planned geothermal facilities.
Here, we present a regional overview of the geochemistry of brines from deep Mesozoic sandstones in the Danish Basin and North German Basin that supplements previous studies, notably by Laier (2002, 2008). The brine composition at shallow burial typically reflects the original (connate) formation water chemistry, which is determined by the original depositional environment of the sandstone, for example fluvial or marine. However, the mineralogical composition of the sandstone changes during burial, whereby some minerals may dissolve or precipitate when exposed to higher temperatures. These mineral changes are reflected in the brine composition, which typically becomes more saline with increased burial (e.g. Laier 2008; Kharaka & Hanor 2003).
The brine chemistry reported here shows a distinct depth trend, which reflects original connate formation waters that are modified through burial diagenesis. We have classified the brines into brine types, which are shown to be related to their depositional environment, depth, geological formation and geographical domains.
Methods
We collected new samples from the production wells at each of the three Danish geothermal sites (Margretheholm, Sønderborg and Thisted) in 2017. The samples were analysed for pH, anions, cations and trace elements. Cations were analysed by ICP-MS (PerkinElmer Elan6100DRC Quadrupol) with a standard deviation of 3–15% depending on the element measured. Samples for anion analysis were frozen for ion-chromatography (LC50-CD50, Dionex, CA, USA) with a quantification limit of 0.05 mg/l. Total dissolved species (TDS) were calculated as the weight sum of analysed ions per weight of saltwater.
These new data are combined with previously published data from the northern North German Basin (Tønder-4, -5 wells, Sønderborg-1A, -2 wells) and from the Danish Basin (Fig. 1, Farsø-1, Aars-1, Stenlille-1, -19 wells, Thisted -2, -3 wells, Margretheholm-1, -2 wells; Laier, 2002, 2008; Hjuler et al. 2019). Collectively, these samples span the Triassic Skagerrak, Bunter Sandstone and Falster formations, the Upper Triassic – Lower Jurassic Gassum Formation and the Jurassic Fjerritslev and Haldager Sand formations (Fig. 2). Contaminated samples picked during test pumping were deselected and only samples in the late phase with stable water chemistry (notably K+ and Cl-) were used. A principal component analysis (PCA) was conducted on the combined dataset to classify the formation water chemistry into one of three brine types (Table 1). Altogether, the new samples and the samples from the literature total 39 samples (Fig. 3). In the PCA, present day vertical depth is used with no correction for Cenozoic uplift.
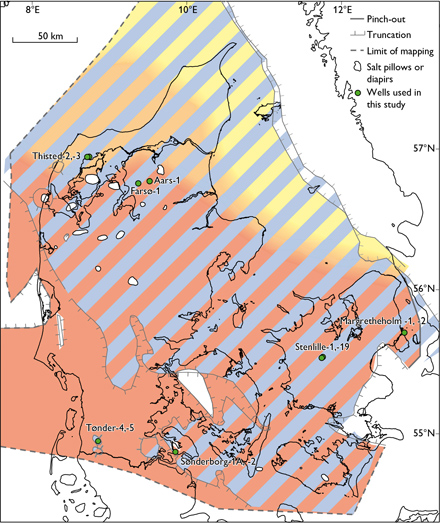
Fig. 1. Location of the wells used for brine type characterisation and distribution of geothermal reservoirs: Skagerrak (yellow – orange), Bunter Sandstone (red), and Gassum (blue) formations. Modified from Weibel et al. (2017a).
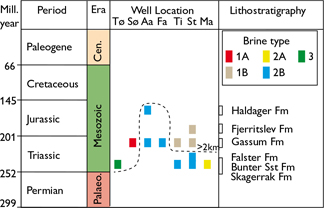
Fig. 2. Stratigraphy of reservoirs and location of brine types. Brine types are defined in Fig. 3. The broken line indicates present-day depth of 2 km below surface. Tø: Tønder. Sø: Sønderborg. Aa: Aars. Fa: Farsø. Ti: Thisted. St: Stenlille. Ma: Margretheholm. Palaeo.: Palaeozoic. Cen.: Cenozoic.
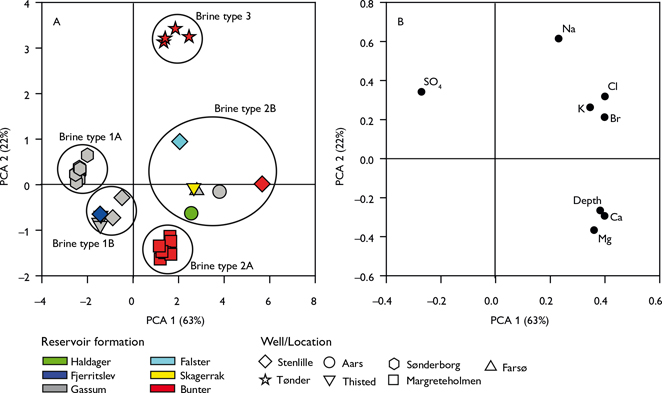
Fig. 3. Principal component analysis (PCA). A: Score plot. B: Loading plot of PCA on regional data. The plots model 85% of the total data variance. Variance proportions are shown along each component axis. Brine types are classified according to their groupings in A.
Results and discussion
To classify the brine types we applied a PCA, which transforms a matrix of measured data (X; comprised of N samples and P variables) into sets of projection subspaces delineated by principal components. Each component is a linear combination of all P variables, which displays variance-maximised interrelationships between the variables (Schovsbo et al. 2016). PCA results are presented as a score plot (Fig. 3A), which displays groupings, or clusters, of samples based on compositional similarities, alongside a loading plot (Fig. 3B), which shows the variable correlations. Finally, we quantify the proportion of the total dataset variance that can be modelled by each component (as shown in each of the axes in Fig. 3).
Brine type classification
In the PCA model, the first two principal component axes resolve 85% of the total data variance (Fig. 3). The main trend expressed on the PCA-1 axis is high ion concentrations in water from deeply buried reservoirs, expressed as high positive PCA-1 loadings at depth (Fig. 3A) for all ions except SO42– (Fig. 3B). The PCA-2 axis displays high positive loadings of Na+, Cl–, K+, SO42– and Br–, as well as high negative loadings of elements like Mg2+, Ca2+ and also at depth (Fig. 3B).
From the PCA, three main brine types can be identified based on natural groupings in the PCA-2 versus PCA-1 plot (Fig. 3A). The characteristics and occurrence of each type are presented below.
Brine type 1 plots with negative PCA-1 and with slightly positive (type 1A) or negative (type 1B) PCA-2 score (Fig. 3A) and is characterised by relative low chloride content (Cl– < 110 000 ppm) and low TDS (Table 1). Type 1A is SO42– enriched, and type 1B is SO42– depleted (Table 1). Type 1 occurs mainly in Gassum reservoirs from the Stenlille, Thisted (type 1B) and Sønderborg wells (type 1A). These three reservoirs are buried between 1.2 and 1.6 km depth.
Brine type 2 is characterised by positive PCA-1 and negative (type 2A) or neutral (type 2B) PCA-2 scores and can be characterised compositionally by medium to high salinities (Cl– >130 000 ppm), and high to very high Ca2+ and Mg2+ concentrations (Fig. 3). Type 2A is Cl– and K+ depleted relative to 2B, whereas type 2B is K+ enriched (Table 1). Brine type 2 occurs at depths greater than 2 km in a broad range of reservoirs belonging to the Haldager Sand, Gassum, Bunter Sandstone and Skagerrak formations. Type 2A occurs exclusively in the Margretheholm area (Fig. 3A).
Brine type 3 has intermediate PCA-1 and highly positive PCA-2 scores (Fig. 3A), reflecting high salinities (Cl– >190 000 ppm, Table 1). Type 3 waters are restricted to the Bunter Sandstone reservoir in the Tønder area overlying a Zechstein salt dome. In addition, halite (NaCl) is also present in the Triassic sequence at Tønder (Laier & Nielsen 1989). The brine is halite saturated and it is estimated that c. 5 g/L halite has precipitated at surface conditions during production tests (Hjuler et al. 2019).
Saturation index from PHREEQC simulations
To further interpret the brine types, we performed a chemical speciation analysis using the numerical code PHREEQC and its Pitzer database. The in situ reservoir temperature was estimated from the regional temperature gradient (e.g. Balling et al. 1981). The fluid pressure of the reservoirs was assumed to be hydrostatic (9.79 KPa/m) assuming a water density of 1.1 g/cm3). The saturation state of the brines with respect to selected minerals is indicated by the Saturation Index (SI) whereby positive and negative values indicate super-saturation and undersaturation, respectively. Equilibrium with respect to the mineral is assumed for –0.4 ≤ SI ≤ +0.4. This accounts for the uncertainties associated with the difficulties of sampling brines at high temperature and pressure, the analytical uncertainty and the application of thermodynamic equilibrium constants on mineral phases in saline systems. Formation water with a SI within this band is assumed to be saturated, and thus in equilibrium for this mineral. Carbonate minerals are not included in Table 2, due to the difficulty of correctly measuring components of the carbonate system (pH, HCO3- etc.) in heated pressurised samples.
The salinity of the brine types increases with the average depth (e.g. Cl– in brine types 1A, 1B, 2B, Table 1). This relationship was previously explained by the diffusion of Cl– from the underlying Zechstein salt deposits occurring in most of the Danish Basin and the North German Basin (Laier 2002). For the deepest reservoirs (type 2B) the brine is saturated with respect to halite (SI = 0.03; Table 2). Even though types 2A and 2B are sampled from the same depth, type 2A has a significantly lower Cl-concentration and is undersaturated with respect to halite (SI = –0.55, Table 2). This may be because brine type 2A, from Margretheholm geothermal plant, was sampled from reservoir sandstones at the basin margin, where Zechstein salt deposits are absent. Brine type 3, sampled in Tønder has a high salt content compared to deeper brines (types 2A and B), and the brine is saturated with respect to halite. Brine type 3 is the only sample located in an area where halite-cemented sandstone intervals are present and salt deposits exist both above (Röth salt) and below (Zechstein salt).
Brine type 3 is saturated with respect to anhydrite (CaSO4 SI = 0.35; Table 2), which makes sense as anhydrite is a common mineral in the Bunter Sandstone Formation (Weibel & Friis 2004). Despite a high SI for barite (BaSO4) in brine type 1A (SI = 0.40, Table 2), we did not observe any barite precipitation in the Margretheholm geothermal plant. The high saturation index probably reflects the uncertainties associated with sampling and analysing from deep reservoirs and most likely the formation water is in equilibrium with barite in the reservoir.
The Ca:Cl ratio (Table 1) is significantly higher in the deeper types (2A and 2B) compared to the shallower brines (types 1A, 1B and 3). Calcite (CaCO3) is common in the shallow part of the Gassum Formation, but ankerite (Ca(Mg, Fe, Mn)(CO3)2) is more abundant in the deeper parts (Weibel et al. 2017b). Hence, replacement of calcite with a Fe and Mgrich carbonate would liberate Ca2+ to the formation water. Similarly, Ca2+ may be liberated to the pore fluid, as dolomite (CaMg(CO3)2) becomes more stable than calcite in the deeply buried parts of the Skagerrak Formation (Weibel et al. 2017a).
The K+ content generally increases with burial depth in the Gassum Formation (Table 1). K+ may have been liberated by albite (NaAlSi3O8) replacement (albitisation) of K-feldspar (KAlSi3O8), which has been documented in Gassum sandstones from both the Aars-1 and Farsø-1 cores (Weibel et al. 2017b). Formation water from the Bunter Sandstone Formation generally has a similarly high or higher K+ content as the deeply buried Gassum Formation. This may relate to a generally higher abundance of K-feldspar and rock fragments (Weibel & Friis 2004), or it may originate from KCl in the under or overlying evaporites.
Conclusions
Here, we have identified and characterised three brine types present in Danish geothermal reservoirs. Reservoir depth and the occurrence of salt in the subsurface layer appear to be a dominant control. Brine type 1 occurs in reservoirs shallower than 2 km, whereas Brine type 2 occurs in both Jurassic and Triassic sandstones buried to more than 2 km. Among the deeply buried reservoirs, type 2A brine is only found in the Margretheholm wells and is interpreted to reflect the absence of salt in the subsurface around these wells in contrast to all other analysed deep wells. Brine type 3 is highly saline and occurs at less than 2 km depth in the Tønder area. Classification of brine types according to chemical composition, highlights variable risks in potential scale and scale types and shows that local conditions must be considered prior to any new planned geothermal facility. Geological information, depth and geographical domains can serve as a rough predictive tool, and will be further refined as additional data are collected from new geothermal wells.
Acknowledgments
This contribution is part of the project GEOTHERM (“Geothermal energy from sedimentary reservoirs – Removing obstacles for large scale utilization”) (No. 6154-00011B) funded by the Innovation Fund Denmark (IFD). We thank the two reviewers, Ida Fabricius and Nicolas Marty, for their comments, which improved the manuscript.
References
Balling, N., Kristensen, J.I., Breiner, N., Poulsen, K.D., Rasmussen, R. & Saxov, S. 1981: Geothermal measurements and subsurface temperature modelling in Denmark. GeoSkrifter 16, 173 pp.
Hjuler, M.L., Olivarius, M., Boldreel, L.O., Kristensen, L., Laier, T., Mathiesen, A., Nielsen, C.M. & Nielsen, L.H. 2019: Multidisciplinary approach to assess geothermal potential, Tønder area, North German Basin. Geothermics 78, 211–223. https://doi.org/10.1016/j.geothermics.2018.12.001
Kharaka, Y.K. & Hanor, J.S. 2003: Deep fluids in the continents: I. Sedimentary Basins. In: Drevor, J.I. (ed.): Treatise on Geochemistry 5, 499–540. https://doi.org/10.1016/b0080437516/05085-4
Laier, T. 2002: Vurdering af udfældningsrisici ved geotermisk produktion fra Margretheholmboringen MAH-1A. Beregning af mætningsindeks for mineraler i saltvand fra Danmarks dybere undergrund. Danmarks og Grønlands Geologiske Undersøgelser Rapport 2002/95. 48pp
Laier, T. 2008: Chemistry of Danish saline formation waters relevant for core fluid experiments. Fluid chemistry data for lab experiments related to CO2 storage in deep aquifers. Danmarks og Grønlands Geologiske Undersøgelser Rapport 2008/48.
Laier, T. & Nielsen, B.L. 1989: Cementing halite in Triassic Sandstone (Tønder Southwest Denmark) as a result of hyperfiltration of brines. Chemical Geology 76, 353–363. https://doi.org/10.1016/0009-2541(89)90103-4
Schovsbo, N.H., Hedegaard, K., Holmslykke, H.D., Kjøller, C., Kristensen, L., Thomsen, E. & Esbensen, K.H. 2016: Formation water and produced water types in Danish oil and gas fields: Implications for enhanced oil recovery by “smart” water. Geological Survey of Denmark and Greenland Bulletin 35, 43–46.
Vosgerau, H. et al. 2016: A WebGIS portal for exploration of deep geothermal energy based on geological and geophysical data. Geological Survey of Denmark and Greenland Bulletin 35, 23–26.
Weibel, R. & Friis, H. 2004: Opaque minerals as keys for distinguishing oxidising and reducing diagenetic conditions in the Lower Triassic Bunter Sandstone, North German Basin. Sedimentary Geology 169, 129–149. https://doi.org/10.1016/j.sedgeo.2004.05.004
Weibel, R. et al. 2017a. The influence of climate on early and burial diagenesis of Triassic and Jurassic sandstones from the Norwegian – Danish Basin. The Depositional Record 3, 60–91. https://doi.org/10.1002/dep2.27
Weibel, R., Olivarius, M., Kristensen, L., Friis, H., Hjuler, M.L., Kjøller, C., Mathiesen, A. & Nielsen, L.H. 2017b: Predicting permeability of low enthalpy geothermal reservoirs: A case study from the Upper Triassic − Lower Jurassic Gassum Formation, Norwegian – Danish Basin. Geothermics 65, 135–157. https://doi.org/10.1016/j.geothermics.2016.09.003
How to cite
Holmslykke, H.D., Schovsbo, N.H., Kristensen, L., Weibel, R. & Nielsen, L.H. 2019: Characterising brines in deep Mesozoic sandstone reservoirs, Denmark. Geological Survey of Denmark and Greenland Bulletin 43, e2019430104. https://doi.org/10.34194/GEUSB-201943-01-04